| Author |
 Topic Topic  |
|
|
ullix
    
Germany
1107 Posts |
|
| Reply #1
the_mike
   
Switzerland
53 Posts |
 Posted - 01/16/2018 : 08:10:17 Posted - 01/16/2018 : 08:10:17


|
Cool - let me know when you go to the potato-chips part ;)
I think GQe should make the potty-training and "going banana" sticky-topics.. @zlm can you help there? |
 |
|
| Reply #2
ullix
    
Germany
1107 Posts |
 Posted - 01/21/2018 : 01:29:45 Posted - 01/21/2018 : 01:29:45


|
Going Cocoa:
Image Insert:
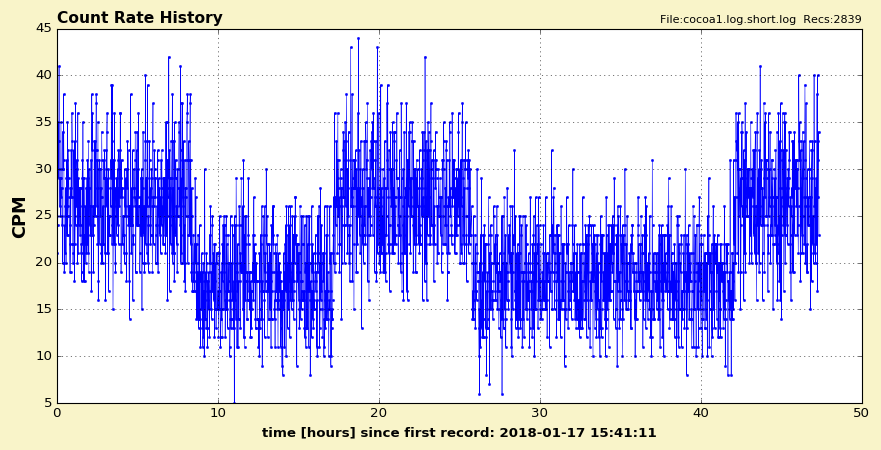
102673 bytes
Continuing the "Going Banana" experiments by measuring household cocoa powder. Same experimental procedure as with the bananas. I think the graph leaves no doubt when the Geiger counter was put on cocoa, and when Background-only was measured.
Huuuh, cocoa is radioactive!
According to this link https://www.jameda.de/naehrstoffe/kalium/ banana have 420 mg Kalium per 100g, while cocoa has 1920 mg K per 100g. This is second only to low fat soy flour at 2015 mg K per 100g. Dried potato products (chips, flour,...) are in the middle with around 1000 mg K per 100g.
Can anyone provide a better measurement of such household items? Perhaps by putting a shield around the setup, could be lead, iron, aluminum?
|
 |
|
| Reply #3
Distelzombie
    
Germany
202 Posts |
 Posted - 01/21/2018 : 09:44:58 Posted - 01/21/2018 : 09:44:58


|
You could extract the potassium. That would give you a way more accurate result.
Here is the method for bananas. h**p://sciencewithryan.blogspot.de/2014/09/33-peels-to-potassium-part-1.html?m=1 (For peels. But you could also burn the whole thing)
|
GMC-300E+ V4.20 with sbt-11a alpha tube
My statements are "stuff-a-hobbyist-says" and not in any way professional. |
 |
|
| Reply #4
ullix
    
Germany
1107 Posts |
 Posted - 01/22/2018 : 02:35:55 Posted - 01/22/2018 : 02:35:55


|
What a neat experiment, thanks for the link.
You will have noticed that this is exactly the original method to produce K that gave "pot ash" its name (which I adapted into my "Potty Training" ). But regarding accuracy you would need to know the preparative losses occurring; not easy.
But I don't want to determine the K content - that is known already - I want to use household and environment items to show that they do contain radioactivity. Though I must admit I expected a more significant effect. You can't really scare anyone by running a 3-day experiment and then use statistical hand wringing to show an enhanced radiative effect.
I am currently running "full potatoes", and I can say while there is an ehancement, it is again of the hand-wringing type.
|
 |
|
| Reply #5
hmw

8 Posts |
 Posted - 01/22/2018 : 11:24:40 Posted - 01/22/2018 : 11:24:40


|
quote:
Originally posted by ullix
But I don't want to determine the K content - that is known already - I want to use household and environment items to show that they do contain radioactivity. Though I must admit I expected a more significant effect.
How about the dust from a windowpane after rain set in after a few dry days? Or the dust from a CRT (if your household still has one)? Or a granite table top? Or the granite paved ways in the next shopping mall? |
 |
|
| Reply #6
Distelzombie
    
Germany
202 Posts |
 Posted - 01/23/2018 : 17:10:43 Posted - 01/23/2018 : 17:10:43


|
I did test the fresh dust from cleaning my CRT once. Here's a picture:
Image Insert:
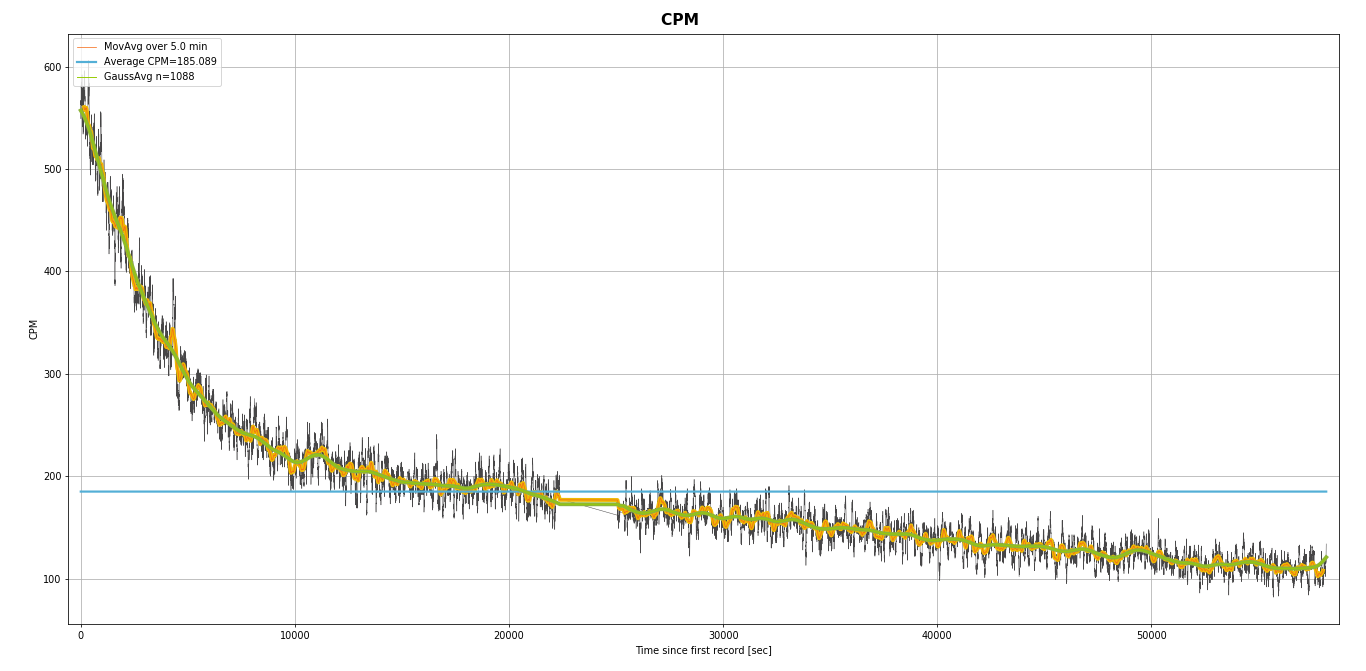
123130 bytes
Anyway. Ullix if you use this method you'll get a better measurement of the radioactivity of a banana or whatever. You just concentrate the potassium. If you simply put your counter on top of a banana you'll lose accuracy due to all the water and other stuff blocking most of the radiation. You'll get a quantitative measurement of all the radioactive stuff in a banana. I think this is the best way.
Except for the extra work. But maybe you want to find the easiest way? If not I don't know why you oppose this
PS: I still like my color scheme better :P Can you change colors in your program? This is an old picture from when I was testing my obsolete program, btw. |
GMC-300E+ V4.20 with sbt-11a alpha tube
My statements are "stuff-a-hobbyist-says" and not in any way professional. |
Edited by - Distelzombie on 01/23/2018 17:12:35 |
 |
|
| Reply #7
ullix
    
Germany
1107 Posts |
 Posted - 01/25/2018 : 02:17:40 Posted - 01/25/2018 : 02:17:40


|
What on earth is your "dust from cleaning my CRT" giving you 600CPM? It would be a tiny amount of dust, hence it would have to have an enormous specific activity? And why does it have a half life of ~2h? Or are there even 2 half lifes? Describe what you did.
And what action am I opposed to? What radioactivity do you expect to see in a banana beyond K40? And to repeat: I am not interested in purifying potassium; I want everyday items, unmodified, which I could use e.g. in classroom demonstrations, easy to understand, easy to grasp, and convincing. KCl as obtainable from garden supply to health food stores fits the bill. Banana does not. Using it was just to meet the challenge of being able to do it at all with a Geiger counter as simple as an GMC300E.
And, look into my Potty Training (page 3) you can get more K40 closer to the counter using KCl than you would using the pure metal. (Ignoring the annoying fact that pure alkali metal would burst into flames.) Potassium Oxide, Carbonate and Sulfate might be even better, but they are harder to come by in suitable form.
Sure, the colors can be changed, like every other detail of the graph, line, marker, font, scale, .... Increases complication significantly with little gain, I think. When I myself need a specific style, e.g. for a publication, I use GeigerLog to determine which data I need, and then import these data into a spreadsheet and format it there as desired.
But if it is important to you, look into script gplot.py, 4 lines from 37...40.
|
 |
|
| Reply #8
ullix
    
Germany
1107 Posts |
 Posted - 01/25/2018 : 02:21:01 Posted - 01/25/2018 : 02:21:01


|
| @hmw: no granite table top available here, but why don't you do it yourself? Likewise the dust from the windowpane etc. Looking forward to your results! |
 |
|
| Reply #9
Distelzombie
    
Germany
202 Posts |
 Posted - 02/08/2018 : 02:02:35 Posted - 02/08/2018 : 02:02:35


|
Dust containing radon does have two half-lifes. One from radon and the other from its doughter.
I don't know why it was so high. I guess it's because it was a relatively thick layer of dust and my counter is sensitive to alpha. Maybe I had my safe in the room at the time, I don't know.
I don't know why you don't understand: if you simply put your counter on a banana you're not measuring the whole amount of K in it.
That's not a good measurement of "amount of radioactivity in my banana". It's just "there is radioactivity in my banana", and we all know that already. Maybe I wasn't clear enough with what I meant. It was not meant as an attack.
Regarding colors: Yeah I know. I didn't mean in code but rather in GUI.
PS: I may sound like an asshole sometimes but that's mostly because I couldn't think of a different way to spell the sentence or I didn't try enough. Sry :) |
GMC-300E+ V4.20 with sbt-11a alpha tube
My statements are "stuff-a-hobbyist-says" and not in any way professional. |
 |
|
| |
 Topic Topic  |
|

